The Institut National du Cancer is a key partner of the National Platform for the Fight Against Cancer. Its overall mission is to fight cancer and ensure the continued development of oncology in Luxembourg by overseeing the implementation of the Cancer Plan so that its goals can be achieved. It will be interdisciplinary and intersectoral as needed.
The INC participated in several working groups, notably the Tumour Board Working Group (WG-TB), whose mission was to structure TB documentation and implement a national IT solution for recording, storing, and analyzing data collected and stored during TB.
The Platform National Cancer (PFN-Cancer) has set up a working group on the “right to be forgotten” in response to axis 9 entitled “patient rights, information, and health democracy” of the National Cancer Plan 2014-2018. The aim of this working group was to ensure that citizens who have had cancer no longer have to mention it when applying for mortgage insurance, and to apply a reference grid for certain types of cancer to the conditions for accepting insurance without additional premiums or exclusions.
Under Luxembourg's Plan National Cancer (PC-Lux) 2014-2018, a national human genetics center was to be created within the LNS.
The law of March 8, 2018, relating to hospitals and hospital planning was published in the Memorial A on March 28, 2018. The hospital plan regulates, in particular, the distribution of services among the various hospitals, allocates them a financial envelope, and sets the number of beds authorized.
The Molecular Diagnosis for Better Cancer Treatment (MDLUX2) program was launched in the summer of 2018 and aims to improve cancer treatment outcomes. It provides Luxembourg patients who need it with specific treatment by giving them access to molecular analysis of their tumours. Patients also have the opportunity to support research by giving informed consent to authorize and allow access to their remaining biological samples and clinical data. This program is expected to benefit up to 200 patients over a three-year period.
The Luxembourg School of Medicine is a work in progress. In simple terms, a “medical school” is a “light” version of a full-fledged medical school. In fact, Luxembourg students currently only have access to a Bachelor's degree in Life Sciences (first year) at the University of Luxembourg (UniLu) and are then forced to continue their studies abroad. The creation of the Luxembourg School of Medicine aims to address this issue and enable Luxembourg to benefit from positive developments in the fields of medicine and research.
In 2018, the INC was invited to participate as a subcontractor and representative for Luxembourg in the work of the European Commission's "Joint Action on Innovative Partnership for Action Against Cancer (iPAAC) " Joint Action of the European Commission, which was selected by the European Commission to open funding under the 3rd Health Program (2014-2020) and brings together a total of 44 partners from 24 European countries. This Joint Action officially started on April 1, 2018, and will continue until 2021.
A national tumour board is a group of experts from different medical specialties, organized at the national level, to discuss complex cases and define the most appropriate treatment strategy for a patient. It promotes collegial decision-making based on best practices and available recommendations.
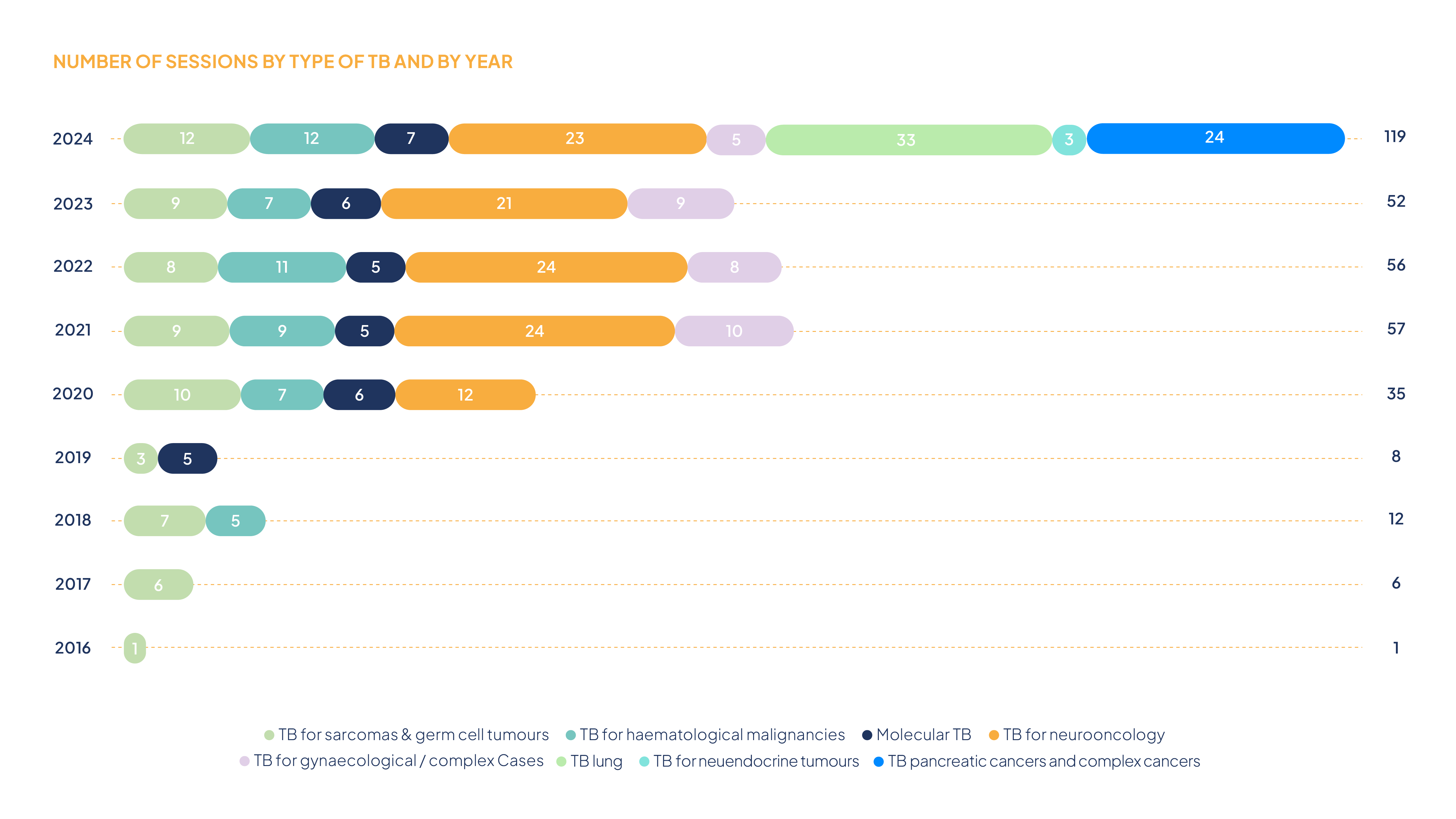
In 2024, the INC organized a total of 119 national TB sessions in accordance with the requirements of the “National Concept for Multidisciplinary Tumour Boards in Oncology,” developed as part of the first National Cancer Plan:

At INC, we actively contribute to organizing updates to patient pathways and guidelines for different types of cancer.
A guideline (G) is a guidance tool developed from national, international, and European guidelines. It is based on a set of recommendations from experts who propose best medical practices, while drawing on publications from scientific research in medicine.
The Patient Pathway (PP) is an indispensable tool for planning and managing care for patients with long-term conditions. It details the different phases of care and serves as a guide throughout the patient's journey. This pathway promotes shared decision-making between the patient and their healthcare team, while providing a clear overview of the steps to be taken and the stakeholders and services involved in the process.
The PP therefore helps to improve the quality and safety of care by harmonizing medical practices at each stage, standardizing interventions in different hospitals, and homogenizing the care provided to patients.
This dual framework, between guidelines and patient pathways, guarantees coordinated, high-quality care focused on the well-being and safety of the patient.

Référentiel national de la prise en charge du cancer du sein (2025)

Référentiel national du cancer de la prostate (2025)

Référentiel national pour le dépistage du cancer du sein en fonction du risque (2025)

Référentiel national en oncologie pulmonaire (2023)

Référentiel national du cancer colorectal (2023)

Référentiel national des mélanomes malins cutanés (2021)

Référentiel national pour le cancer du système nerveux central (2020)

Référentiel national pour les cancers hématologiques (2019)

Référentiel national pour le cancer du pancréas (2019)

Référentiel national pour le parcours des patients pour le cancer du poumon (2018)

Référentiel pour les cancers gynécologiques (2018)

Standardised patient pathway for breast cancer (2025)

Subprocess for standardised patient pathway for prostate cancer (2025)
Standardised patient pathway for prostate cancer (2025)
Subprocess for standardised patient pathway for prostate cancer (2025)
Cancer colorectal - consultation du patient (2023)
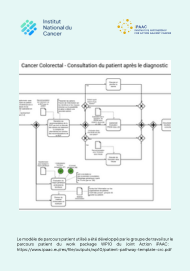
Cancer colorectal - consultation du patient après le diagnostic (2023)

Parcours standardisé pour le cancer colorectal (2023)
The annual activity reports of the Institut National du Cancer (INC) in Luxembourg provide a comprehensive overview of the actions carried out each year. They highlight key initiatives, national, European, and international collaborations, as well as progress made under the second Plan National Cancer. These documents reflect the INC's commitment to improving public health and strengthening the fight against cancer in Luxembourg.
The INC's annual reports are published as part of the official activity reports of the Luxembourg Ministry of Health.
Stay informed about the latest actions, publications, projects and events organized by the Institut National du Cancer in Luxembourg.
Our news reflects our day-to-day commitment to improving cancer prevention, support and information.
Read moreScroll